Past Webinars

Helmut Hiller, MS & Maria Beery, MS
September/2024
nPOD Data Portal
View Details
 DATE: Friday, September 6th, 2024
DATE: Friday, September 6th, 2024
TIME: 11:00AM EST
Meeting Recording: Dropbox link to view recording
Discussion led by: Helmut Hiller, MS & Maria Beery, MS
In this webinar, we guided you through the nPOD Data Portal website, and showed you how to navigate, access, and download our new genetic data, including SNPs-genes and immunophenotyping data. We also demonstrated how to use our histopathology online page to search for samples, identify study cases, and efficiently prepare and submit requests to the nPOD team.

Maria Beery, MS & Irina Kusmarteva, PhD
September/2022
Data Portal
View Details
DATE: Thursday, September 22nd, 2022
TIME: 11:00am – 012:00pm EST
Download Meeting Recording: Dropbox link to view meeting recording
Discussion led by: Maria Beery, MS & Irina Kusmarteva, PhD

Romano Regazzi & Alberto Pugliese
August/2022
Small RNA molecules (miRNAs, circRNAs, tRNAs, and more): relevance to Type 1 diabetes
View Details
DATE: Thursday, August 7, 2022
TIME: 11:00am – 1:00pm, EST
Meeting Recording: Dropbox link to view meeting recording
Discussion led by: Romano Regazzi, PhD and Alberto Pugliese, MD
nPOD Investigators
July/2021
Innate Immunity in Type 1 Diabetes in nPOD Donors
View Details

DATE: Thursday, July 22nd, 2021
TIME: 11:00am – 01:00pm EST
Download Meeting Recording: Dropbox link to view meeting recording
Discussion led by: Alberto Pugliese, MD, Sally Kent, PhD, Hubert Tse, PhD, Bruce Verchere, PhD, Xiangwei Xiao, MD, PhD and Rachel Friedman, PhD
Last December, we held a stimulating webinar on the role of macrophages in type 1 diabetes. A number of people have since expressed interest on the topic, and it has been suggested to examine more broadly the contribution of the innate immune system to the pathogenesis of type 1 diabetes. Building on the above, we would like to organize a follow-up webinar discussion possibly leading to the organization of a collaborative working group. The group could conceive an experimental strategy for coordinated investigations of nPOD donors and apply innovative study methodologies.
Pancreas Slice Group Webinar
July/2021
nPOD Pancreas Slices Group Immunology
View Details
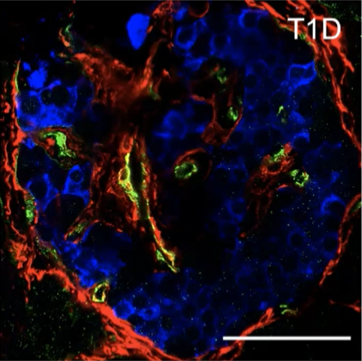
Pancreas Slice Group Webinar
July/2021
nPOD Pancreas Slices Group: Islet Biology Part 2
View Details

DATE: Wednesday, July 14th, 2021
TIME: 11:00am – 01:00pm EST
A webinar session to discuss emerging results from nPOD investigators:
Ricardo Pastori/Juan Dominguez-Bendala
Richard Benninger
Emily Sims and the Pro-Hormone Working Group
Hubert Tse/Jared Taylor
Matthias von Herrath/Mehdi Benkahla
Pancreas Slice Group Webinar
July/2021
nPOD Pancreas Slices Group: Islet Biology Part 1
View Details

DATE: Thursday, July 8th, 2021
TIME: 11:00am – 01:00pm EST
Discussion led by: Irina Kusmartseva, PhD, Maria Beery, MS, and Helmut Hiller, MS

T1D BBQ: The Big Burning Questions Webinar
December/2020
Macrophages in Type 1 Diabetes
View Details
DATE: Thursday, December 10th, 2020
TIME: 11:00am – 01:00pm EST
Download Meeting Recording: Dropbox link to view meeting recording
Discussion led by: Hubert Tse, PhD, Joana Collares Pereira Almaca, PhD, Xiangwei Xiao, MD, PhD, and Bruce Verchere, PhD

Pancreas Slice Group Webinar
May/2019
nPOD Pancreas Slice Group Discussion
View Details
DATE: Wednesday, May 22nd, 2019
WEBINAR INFO:
Click here to join WebEx Meeting
Meeting Number (access code): 737 898 840
Meeting Password: xhMMM3CM
Call-in toll number (US/Canada): 1-650-479-3208
Global Call-in Numbers
Can’t join the meeting?
TIME: 11:00am – 02:00pm EST
Discussion led by: Alberto Pugliese, MD

Alberto Puglese, MD and Sally Kent, PhD
April/2019
Autoimmunity Group Webinar
View Details
DATE: Thursday, April 25th, 2019
Download Meeting Recording: Dropbox link to view meeting recording
Discussion led by: Alberto Pugliese, MD and Sally Kent, PhD
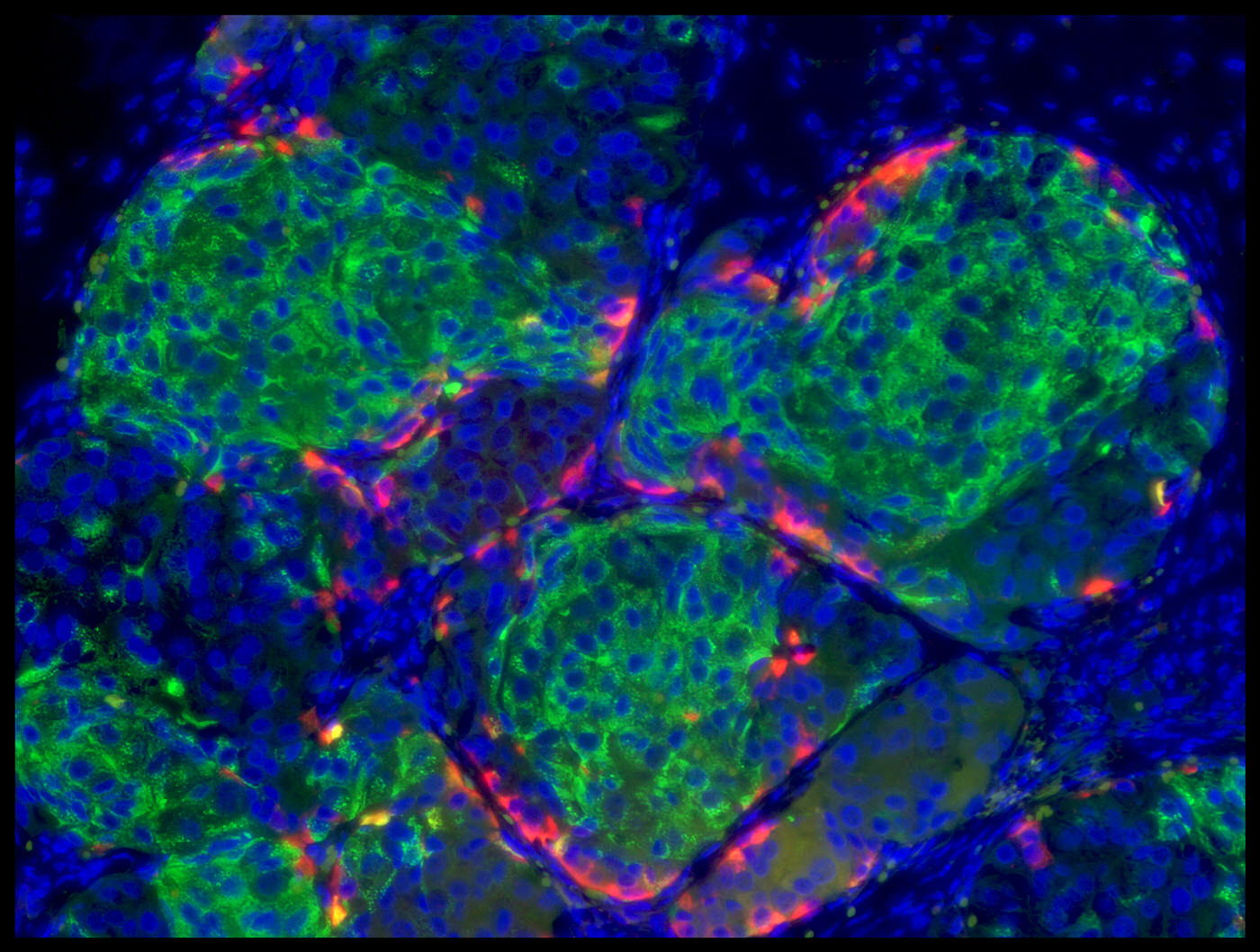
RFA for nPOD Pancreas Slice Program
April/2019
nPOD Islet Dysfunction Group and Exocrine Function Group Slice RFA Webinar
View Details
DATE: Friday, April 26th, 2019
Download Meeting Recording: Dropbox link to view meeting recording
Discussion led by: Stefan Speier, PhD and Clayton Mathews, PhD

RFA for nPOD Pancreas Slice Program
April/2019
nPOD-Autoimmunity Group Slice RFA Webinar
View Details
DATE: Wednesday, April 24th, 2019
Download Meeting Recording: Dropbox link to view meeting recording
Discussion led by: Alberto Pugliese, MD and Sally Kent, PhD

RFA for nPOD Pancreas Slice Program
April/2019
nPOD-Extracellular Matrix Group Slice RFA Webinar
View Details
DATE: Thursday, April 25th, 2019
Download Meeting Recording: Dropbox link to view meeting recording
Discussion led by: Alberto Pugliese, MD and Marika Bogdani, PhD
RFA for nPOD Pancreas Slice Program
April/2019
nPOD-Virus Group Slice RFA Webinar
View Details
DATE: Tuesday, April 23rd, 2019
Download Meeting Recording: Dropbox link to view meeting recording
Discussion led by: Alberto Pugliese, MD and Sarah Richardson, PhD
nPOD Investigators, Group Presentation
October/2017
nPOD Case Deep Dive: #6243
View Details
DATE: Tuesday, October 24th, 2017
SPEAKERS: nPOD Investigators, group discussion led by Alberto Puglese, MD
Click here to view a recording of the webinar.
The nPOD “Deep Dive” Case Study webinar series aims to unite the efforts, expertise, and resources of various Investigators within the nPOD cohort in order to paint a uniquely detailed and comprehensive picture of the development of type 1 diabetes in individual donors of interest. By “comparing notes” in this way, we believe this webinar series will provide a unique opportunity for a deep analysis of individual nPOD donors, based on the synthesis of each presenter’s area of expertise. Not only will this series allow each Investigator to add their “part” to the “whole”, it is also our hope that each nPOD Investigator will be able to walk away from the webinar with new insights from other studies that will inform their research going forward.
This series will begin with the well-studied nPOD Case #6243. This donor was a 13-year-old male who had T1D for 5 years, was mIAA+, and as a “typical” T1D case with both insulitis and insulin-positive islets, will be a fantastic starting place for such an in-depth and collaborative analysis.
All nPOD Investigators are invited to present 1-3 slides of data they may have on Case #6243. If you are unable to attend the webinar in person, we ask that you would still consider sending any data you have on this case to be presented on your behalf. Everyone is invited to attend the webinar, even if your research has not examined this particular case! A recording of the webinar will be made available for those unable to attend.
If you are able to provide data for nPOD Case #6243, please email slides to Joseph Pobee by Friday, October 13th.

Sally Kent, PhD & Maki Nakayama, MD, PhD
January/2017
Islet-Infiltrating T Cell Repertoire: Two Studies
View Details

DATE: Wednesday, January 11th, 2017
SPEAKERS: Sally Kent, PhD
University of Massachusetts Medical School
Maki Nakayama, MD, PhD
University of Colorado
Click here to view a recording of the webinar.
Broad Repertoire of Autoreactive T Cells from the Islets of Donors with Type 1 Diabetes:
In this webinar, we will be discussing our recent study on the analyses of live, autoreactive CD4 and CD8 T cells derived directly from the islets of donors with type 1 diabetes (Babon et. al., Nature Medicine, 2016, PMID: 27798614). We will be discussing the islet samples, handling of the islets, isolation and growth of T cells from the islets, and analyses of their autoreactivity and function to known islet peptide targets and to modified peptides. We will also discuss current and future studies in these ongoing analyses.
Islet-Derived CD4 T Cells Targeting Proinsulin in Human Autoimmune Diabetes:
Type 1 diabetes results from chronic autoimmune destruction of insulin-producing beta cells within pancreatic islets. While insulin is a critical self-antigen in animal models of autoimmune diabetes, due to extremely limited access to pancreas samples, little is known about human antigenic targets for islet-infiltrating T-cells. In this webinar, we will show that proinsulin peptides are targeted by islet-infiltrating T-cells from type 1 diabetes patients. We identified hundreds of T-cells from inflamed pancreatic islets of three young type 1 diabetes organ donors with a short disease duration with high risk HLA genes using a direct T-cell receptor (TCR) sequencing approach without long-term cell culture. Among 85 selected CD4 TCRs tested for reactivity to preproinsulin peptides presented by diabetes-susceptible HLA-DQ and -DR molecules, one T-cell recognized C-peptide amino acids 19-35, and two clones from separate donors responded to insulin B chain amino acids 9-23 (B:9-23) which is known to be a critical self-antigen driving disease progress in animal models of autoimmune diabetes. These B:9-23-specific T-cells from islets responded to whole proinsulin and islets, while previously identified B:9-23 responsive clones from peripheral blood did not, highlighting the importance of proinsulin-specific T-cells in the islet microenvironment.
Maria Beery, MS & Irina Kusmartseva, PhD
November/2016
Maximizing Your nPOD Sample Request: Using Online Pathology and DataShare to Select the Right Cases for Your Study
View Details
DATE: Wednesday, November 30th, 2016
SPEAKERS: Maria Beery, MS
nPOD Senior Biological Scientist
Irina Kusmartseva
nPOD Organ Processing and Pathology Core (OPPC) Director
Download PowerPoint presentation.
As any veteran nPOD investigator knows, every donor case is different. While it is tempting to order based on donor classification alone, we encourage our investigators to be highly selective when requesting material for their studies and to take into consideration both the donor’s clinical history and histopathology report. All nPOD case information is compiled in two distinct databases, Online Pathology and DataShare.
Our Online Pathology database, hosted by Aperio eSlide Manager, displays basic donor demographics alongside high quality scanned images of pancreas, spleen, lymph node, and duodenum sections. While not every sample type will be available for every case, several available blocks are screened by H&E and stained for insulin, glucagon, CD3, and other pertinent markers. In the event that insulitis is found in Type 1 cases, all available pancreas blocks are screened. Viewing images in Online Pathology gives you the ability to make informed decisions about which sample blocks are most relevant to your study.
Our second database, DataShare, supplies an abundance of clinical data, shared nPOD investigator data, and access to our sample request system. Using DataShare in conjunction with Online Pathology allows you to match cases of interest by age, gender, autoantibody status, BMI, and a host of other variables while viewing images from available samples at the same time. In this webinar, the nPOD OPPC team will provide guidance and general tips for navigating both databases and ordering the samples that best suit your needs.

Laura Jacobsen, MD
July/2016
Venue for Highlighting Unique nPOD Cases: Clinical Images in Diabetes
View Details

DATE: Tuesday, July 26th, 2016
SPEAKER: Laura Jacobsen, MD
University of Florida
Click here to view a recording of the webinar.
The nPOD database offers a wealth of knowledge; consolidating a large series of cases and slides into a collective that provides valuable data towards common features for the disorder’s pathogenesis. However, extensive information also exists in individual cases, a feature that can offer unique insights into disease pathophysiology at a personalized level. These latter findings can be presented in a brief, case-based report focused around images, specifically using nPOD histological images.
The Clinical Images in Diabetes section within the journal Diabetes Care represents an exciting, new (July, 2016) forum for conveying case-reports. This section, developed in part with guidance from nPOD, is almost tailor-made for reporting nPOD cases. Indeed, two nPOD cases have already been accepted for publication and others are being considered. First, was nPOD case 6196, looking at a clinical case of type 1 diabetes (T1D) with positive GAD autoantibodies, but contradictory histology (numerous insulin-positive islets and amyloid present) (Diabetes Care 2016 Jul; 39(7): 1292-1294). The second report, nPOD case 6263, is in press and focuses again on contradictory findings between clinical/laboratory review and histological features. This case was also analyzed further in the setting of ethnicity as well as genetic findings of yet to be determined significance. These cases were chosen after our team, made up of pediatric endocrinologists and pathologists, reviewed the donor charts and available laboratory data in conjunction with the histological images and there were unexpected findings or unique characteristics. These cases, their reviewer critiques and ways to get started will be presented, as well as the criteria set forth by Diabetes Care for this Clinical Images in Diabetes section, to encourage nPOD investigators to, in like form, present additional interesting cases from the nPOD collection. This, for the purpose of furthering the notion of disease heterogeneity in T1D as well as to provide novel insights towards the various pathogenic mechanisms leading to diabetes.
REFERENCES:

Alex Marson, MD, PhD
April/2016
Genetic and Epigenetic Fine-Mapping of Causal Type 1 Diabetes Risk Variants
View Details

DATE: Tuesday, April 26th, 2016
SPEAKER: Alex Marson, MD, PhD, University of California, San Francisco
Click here to view a recording of the webinar.
Type 1 diabetes (T1D) has been the subject of intensive genetic investigations, including genome-wide association studies (GWAS) that have collectively identified over 40 risk loci in the genome. Our knowledge of the genetic code provides a powerful framework to understand the deleterious effects of variants that alter protein sequence. However, almost 90% of common variants associated with T1D and other autoimmune diseases fall outside of genes, in non-coding, regulatory regions of the genome—regions previously described as ‘junk DNA.’ We still do not know how DNA variation throughout the genome, especially in non-coding regions, affects cellular pathways and contributes to T1D. We need to develop sophisticated analytic and experimental tools to delve deeper into the function of these non-coding genome elements.
We recently leveraged the latest high-density genotyping data on patients with autoimmune diseases to develop a new algorithm to fine-map the specific variants most likely to cause increased risk of disease. To interpret the biological processes affected by candidate non-coding variants, we generated a large epigenetic resource of cis-regulatory maps of specialized human cells. Comparing SNP locations with chromatin maps for 56 cell types revealed the cell type-specificities of cis-regulatory elements that coincide with fine-mapped risk for T1D and other autoimmune diseases, thus predicting pathogenic cell types. While the primary signature of T1D risk variants is in immune cell enhancers, we also found enrichment in pancreatic islet enhancers.
Moving forward, we will collaborate with nPOD to test the in vivo effects of the variants on chromatin state and gene expression in a population-based study of genotyped T cell samples sorted directly from the pancreatic lymph nodes of human organ donors with and without T1D. Understanding how causal non-coding T1D risk variants disrupt key gene programs controlling cell function will provide critical information linking genetic variation to cellular pathways. This knowledge could accelerate development of targeted therapeutic approaches.
- REFERENCES: Farh and Marson et al. (2015) Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature, 518, 337-343.

Erik Yusko, PhD & Todd Brusko, PhD
December/2015
Immunosequencing: Generating a Potential New Class of Diagnostics
View Details
DATE: Tuesday, December 15th, 2015
SPEAKER: Erik Yusko, PhD, Adaptive Biotechnologies
Todd Brusko, PhD, University of Florida
Click here to view a recording of the webinar.
The cellular and humoral adaptive immune system generates a remarkable breadth of diversity of antigen receptors by combinatorial shuffling of gene segments in somatic cells. The diversity of possible receptors is large and until recently this diversity precluded the possibility of capturing the antigen receptor repertoires. We have developed a method for unbiased amplification of rearranged T-cell and B-cell receptor variable regions and the use of high throughput sequencing to capture millions of these sequences. We have applied the technology to a variety of clinical problems including hematological malignancies, solid tumor immunology, and the immune response to pathogenic stimulation. The technology is used clinically in Adaptive Biotechnologies’ CLIA lab for diagnosis/patient stratification for leukemia and lymphoma, monitoring minimal residual disease, and measuring immune reconstitution after transplant. For application to solid tumors, infiltrating lymphocyte count and clonality are both predictive of response to immunotherapy and prognostic of clinical outcome with standard treatment. Preliminary results from TCRB profiling of various tissues from nPOD donors will also be presented.
REFERENCES:
- PairSEQ: http://stm.sciencemag.org/content/7/301/301ra131
- Reading Immunological Memory: http://biorxiv.org/content/early/2015/09/11/026567
- PCR Bias Control: http://www.ncbi.nlm.nih.gov/pubmed/24157944